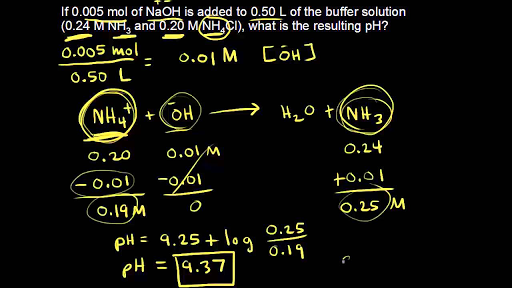
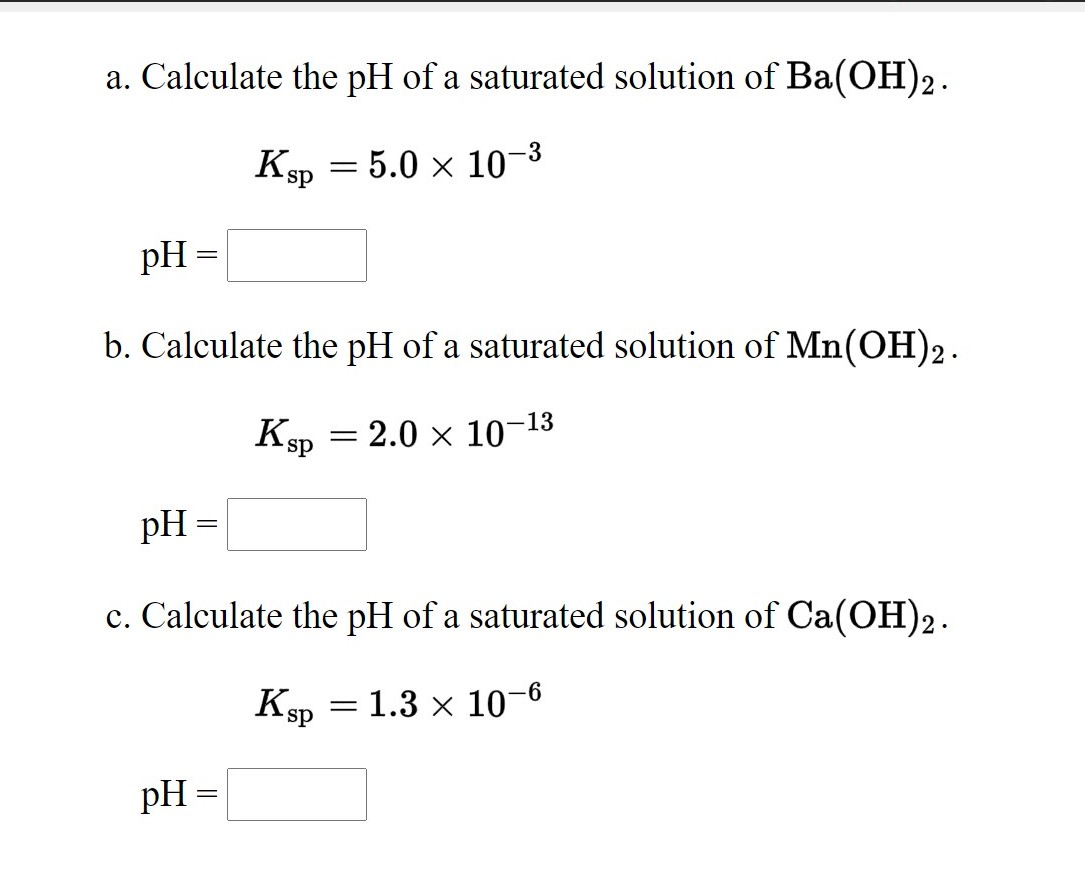
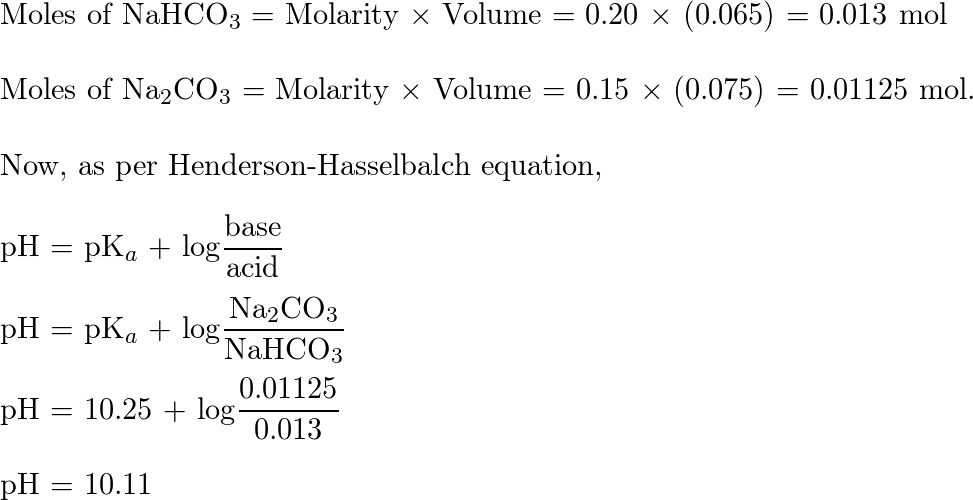Calculate the pH of a solution obtained by mixing 5 mL of 0.1 M NH(4) OH with 250 mL of 0.1 M NH(4) Cl solution . K(b) for NH(4)OH=1.8xx10^(-5).
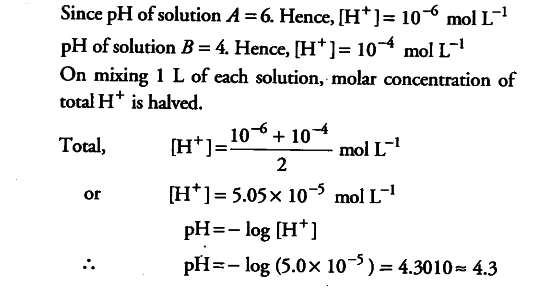
Calculate the pH of a solution formed by mixing equal volumes of two solutions, - CBSE Class 11 Chemistry - Learn CBSE Forum

SOLVED: Calculate the pH of a solution that contains 3.9 × 10-9 M H3O+ at 25°C. Calculate the pH of a solution that contains 3.9 × 10-9 M H3O+ at 25°C. 9.41 3.51 4.59 8.41 5.59

pH, pOH, H3O+, OH-, Kw, Ka, Kb, pKa, and pKb Basic Calculations -Acids and Bases Chemistry Problems - YouTube
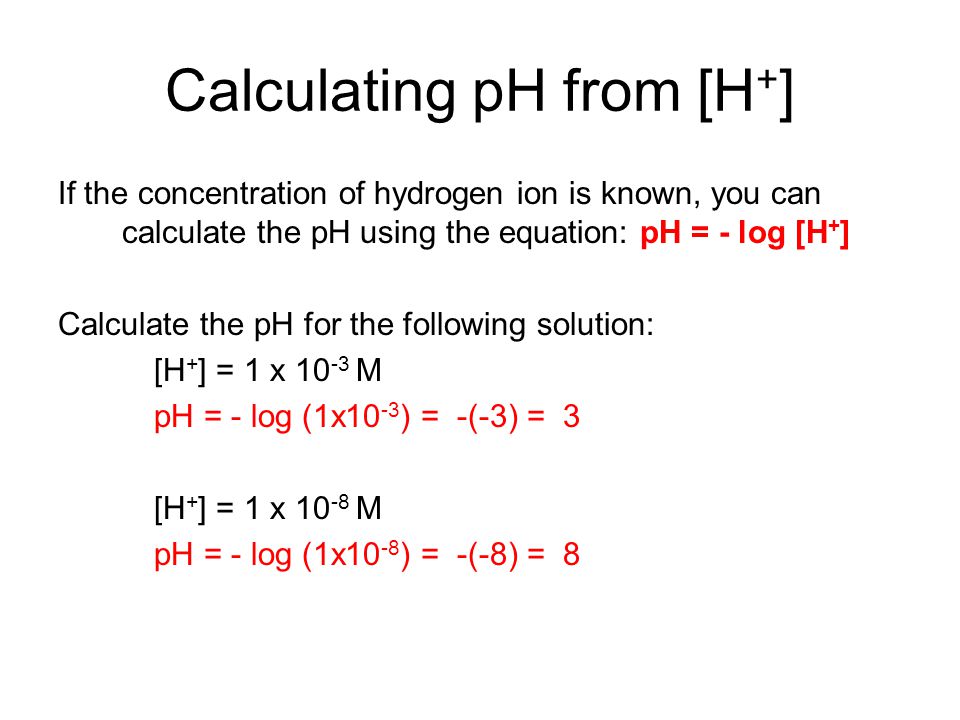



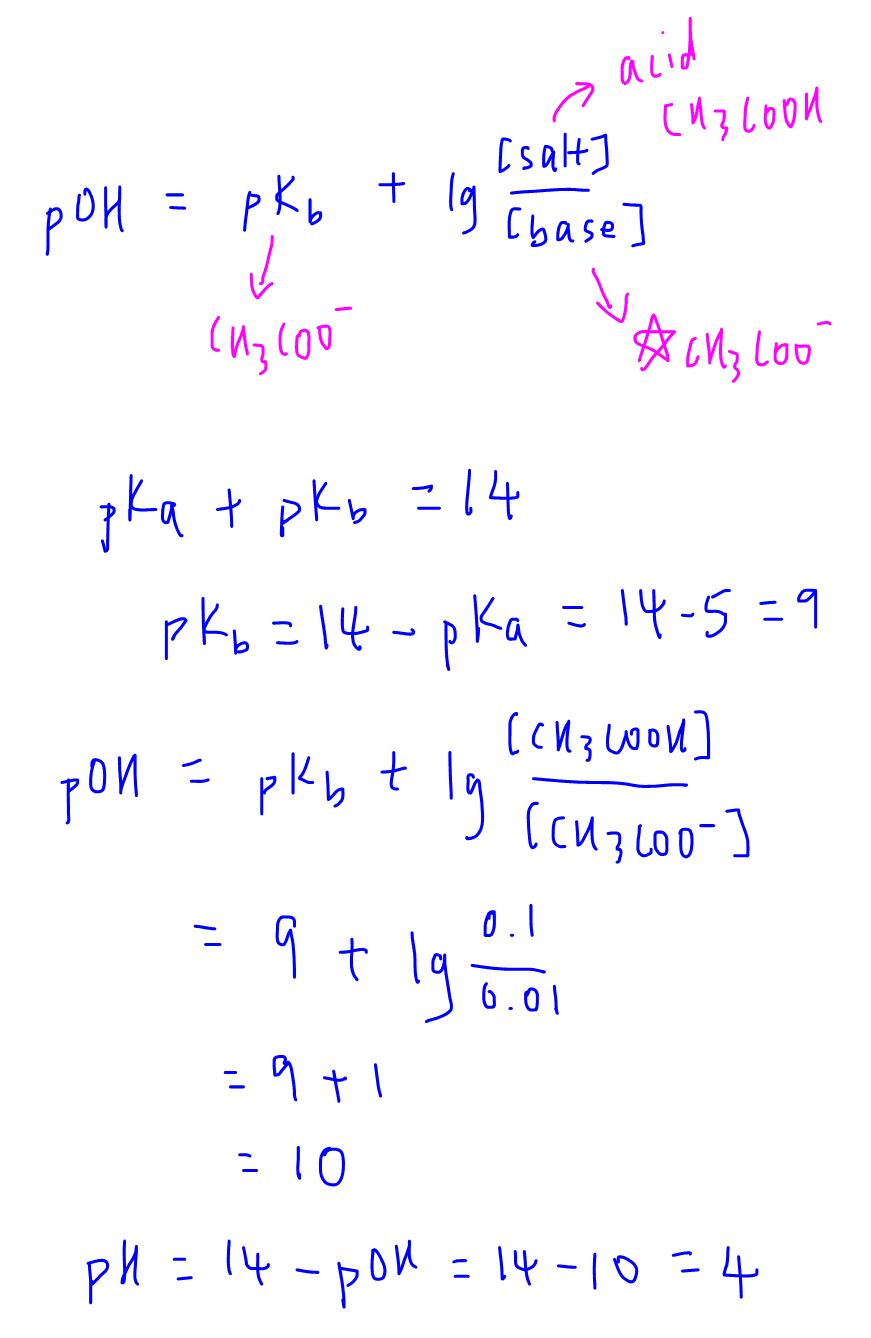
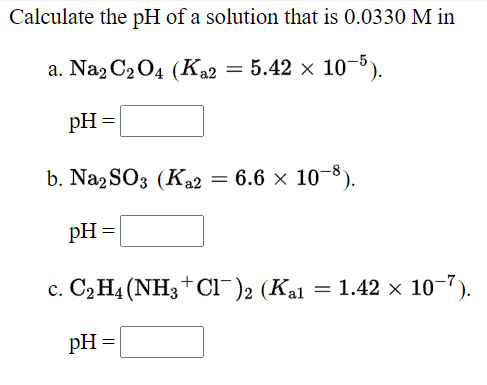



![Given [H+] or [OH-], Calculate pH & pOH - YouTube Given [H+] or [OH-], Calculate pH & pOH - YouTube](https://i.ytimg.com/vi/ghIYaqo0Ycc/maxresdefault.jpg)